
Information for the General Public
- What is mpox?
- What are the symptoms of mpox?
- How can mpox spread?
- Are young children at risk for mpox?
- What do you need to know about mpox if you are a teen or young adult?
- What if I am concerned I may have mpox?
- What if I or my partner have mpox?
- Is there any treatment for mpox for children?
- Where can I find vaccines?
- Resources
Information for Healthcare Providers
- Clinical signs and symptoms
- Infection Control
- Reporting
- Laboratory testing
- Vaccines for pre- & post-exposure prophylaxis
- Treatment
- Is there a mpox vaccine indicated for use in children and how do I obtain it for my patients?
- How can I protect myself and other members of the practice team from mpox?
- Is post-exposure prophylaxis recommended for health care workers who have been exposed to mpox?
- What is the guidance for newborns in hospitals who may have been exposed to mpox during and after delivery?
Effective 6/1/2024, the mpox dashboard has been discontinued. Orange County Health Care Agency (OCHCA) continues to investigate and conduct contact tracing on all confirmed and probable cases and continues to monitor and coordinate post-exposure prophylaxis for high-risk close contacts to known cases. The risk of mpox in the general population remains very low based on current information available. For State updates, please visit the CDPH mpox data dashboard.
Information for the General Public
What is mpox?
- Mpox (formerly known as monkeypox) is a rare disease that is caused by the mpox virus.
- Mpox virus belongs to a group of viruses called Orthopoxvirus, which includes the variola (smallpox) virus, and the vaccinia virus, which is used in the smallpox vaccine.
- Mpox is of public health concern because the illness is like smallpox and can be spread from infected people, animals, and items contaminated with the virus (ex: bedding sheets).
- Mpox was first identified in 1958 and occurs primarily in Central and West African countries.
- Mpox cases have occurred in the U.S. in the past (mostly related to international travel or importation of animals) but have been very rare.
- Recently people with mpox infection, not related to travel to Central and West Africa, have been reported in multiple countries, including the US.
What are the symptoms of mpox?
- Early flu-like symptoms of mpox can include:
- Fever
- Headache
- Muscle aches and backache
- Swollen lymph nodes
- Chills
- Exhaustion
- A rash or sore, sometimes located on or near the genitals or anus, but sometimes in other areas like the hands, feet, chest or face.
- Sores will go through several stages before healing.
- Sores may be inside the body, including the mouth, vagina, or anus.
- Some people experience a rash or sores first, followed by other symptoms and some only experience a rash or sores.
- Mpox can be spread from the time symptoms start until all sores have healed and a fresh layer of skin has formed – this can take several weeks.
How can mpox spread?
- Direct skin-to-skin contact with the sores or scabs of people with mpox
- Direct contact with body fluids of people with mpox, such as drainage from skin sores or saliva that was in contact with mouth sores
- Contact with the respiratory secretions of people with mpox, such as saliva, during prolonged, face-to-face contact or during intimate physical contact, such as kissing, cuddling, or sex
- Touching items (such as bedding towels, clothing, cups and utensils) that previously touched the sores or body fluids of people with mpox
To date, there has been no evidence that mpox is spread by:
- Attending an outdoor event with fully clothed people
- Trying on clothes or shoes at a store
- Traveling in an airport, on a plane or on other public transit
- Swimming in a pool or body of water
- Casual contact with other people
Are young children at risk for mpox?
The risk of mpox, to the general population is low. There are many rash illnesses in children some can look like mpox. If you are concerned about your child’s health talk to your healthcare provider. Testing for mpox is widely available through your healthcare provider.
What if I am concerned I may have mpox?
Public Health recommends that you speak to your primary care provider. Most providers can now do testing for monkeypox through commercial laboratories.
If you do not have a regular provider, call 2-1-1 or the Orange County Health Care Agency's Health Referral Line at 800-564-8448 for assistance.
What if I or my partner have mpox?
- Follow the treatment and prevention recommendations of your healthcare provider.
- If you have mpox or are waiting for test results please see CDC’s Mpox Home Isolation Guidance for the General Public.
Is there any treatment for mpox for children?
Most people recover in two to four weeks even without medicines that kill the virus causing mpox. Your child may need treatment if they have complications or severe disease or are at high risk for severe disease. Treatment may be advised if they have lesions on certain parts of their body (for example, eyes, mouth, genitals, or anus).
Mpox remains contagious until the rash is completely gone---after all scabs have fallen off and new skin has formed. Parents and caregivers of children with mpox should:
- Cover the child’s skin rash.
- Remind their child to avoid scratching or touching the rash or eyes.
- Keep other people and pets away from the child. If possible, one person should provide all care for them.
- Have the child wear a well-fitting mask if they are 2 years old or older when others are taking care of them. The caregiver should wear a respirator or well-fitting mask and gloves when touching the child and handling bandages or clothing.
- Keep the child isolated and home from school or other activities until they are no longer contagious.
Where can I find vaccines?
More information on availability can be found here.
Resources
Information for Healthcare Providers
Clinical signs and symptoms
The CDC has put together a Mpox Clinical Recognition page, that provides information on incubation period, prodromal symptoms, provides pictures of characteristic rash, and the time-course of the rash.
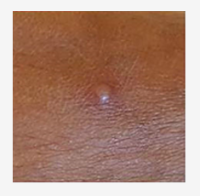

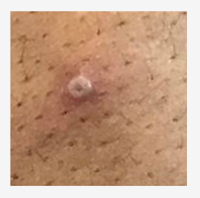
Photo source: UK Health Security Agency
For additional information, please see the Infectious Disease Society of Webinar for providers from July 23, 2022: Mpox: Updates on Testing, Vaccination and Treatment.
Infection Control
For the most current recommendations see the CDC’s Mpox Infection Control: Healthcare Settings.
Personal protective equipment used by healthcare personnel who enter the patient’s room should include:
- Gown
- Gloves
- Eye protection (i.e., goggles or a face shield that covers the front and sides of the face)
- NIOSH-approved N95 filtering facepiece or equivalent, or higher-level respirator
Reporting
If you suspect that you have a patient with mpox contact the OCHCA Communicable Disease Control Division immediately at 714-834-8180.
Testing
There are now multiple commercial laboratories offering PCR testing for mpox, including Quest Diagnostics, Labcorp, Aegis Sciences, and Mayo Clinic Laboratories. Healthcare providers should submit specimens through commercial laboratories if possible, as these labs are likely to provide results with a shorter turnaround time. Public Health is also offering PCR testing for providers who do not have access to commercial laboratory testing or for patients who do not have insurance. Requests for testing must be preapproved by calling the OCHCA Communicable Disease Control Division immediately at 714-834-8180.
Specimen collection requirements
- The following human specimens may be submitted for testing at CDPH VRDL: Dry swabs of lesions, using sterile nylon, polyester, or Dacron swabs with plastic or aluminum shaft.
- More than one lesion should be sampled, preferably from different body sites, for preliminary and confirmatory testing.
- Vigorously swab or brush lesion with two separate sterile dry swabs;
- Break off swabs into separate 1.5- or 2-mL screw-capped tubes with O-ring or place each entire swab in a separate sterile container.
- Label sample and store each lesion separately.
- Acceptable specimen types include lesion swabs (dry or in viral transport medium and lesion crusts. Swabs are preferred over lesion crusts. At this time universal transport media is not acceptable for specimen submission.
- Store all specimens at 4°C if shipping within 24-72 hours; store at -80C if shipping will be delayed.
Vaccines for pre- & post-exposure prophylaxis
When used properly vaccination can be effective at preventing or mitigating mpox virus infection. Resources on the use of vaccines are linked below as well as links to ACIP guidance on the use of the two licensed vaccines (ACAM200 and JYNNEOSTM ) to prevent smallpox as well as links to the package inserts for these products.
- Vaccine availability in Orange County
- CDC Mpox Vaccine Guidance
- Use of JYNNEOS (Smallpox and Mpox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022 MMWR / June 3, 2022 / Vol. 71 / No. 22
- Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses – Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015 Source: MMWR 2016, 65(10);257–262
- ACAM2000, (Smallpox (Vaccinia) Vaccine, Live) package insert
- JYNNEOS (Smallpox and Mpox Vaccine, Live, Nonreplicating) suspension for subcutaneous injection package insert
Treatment
Most cases of mpox experience mild or self-limited diseases. There is currently no licensed treatment for monkeypox. See the CDC’s Treatment Information for Healthcare Professionals for the latest guidance.
Tecovirimat
Tecovirimat (also known as TPOXX or ST-246) is FDA-approved for the treatment of human smallpox disease caused by Variola virus in adults and children. Tecovirimat may be considered for treatment in people infected with mpox virus who either 1) have severe disease, 2) are at high risk of severe disease, or 3) have infection of anatomical areas which may pose a special hazard, such as eyes, mouth, genitals, or anus.
CDC and FDA have developed a process for healthcare providers to provide tecovirimat (TPOXX) treatment to patients with mpox under the expanded access investigational new drug (EA-IND). Additional information for providers interested in obtaining tecovirimat for mpox treatment can be found here.
Is there a mpox vaccine indicated for use in children and how do I obtain it for my patients?
There is currently no mpox vaccine available for administration to all children. However, there is a vaccine available to children < 18 years who have been exposed to mpox. JYNNEOS vaccine may be recommended for children <18 years of age for post-exposure prophylaxis under a single patient expanded access investigational new drug (IND) protocol through CDC. Clinicians should discuss use of vaccine in a child as post-exposure prophylaxis with the state or local health department and CDC.
How can I protect myself and other members of the practice team from mpox?
Currently, vaccination is not recommended for most health care workers. CDC recommends that people whose jobs (clinical or research laboratories and certain health care and public health team members) may expose them to orthopoxviruses, such as mpox, receive either JYNNEOS or ACAM2000 vaccine.
Health care workers should utilize the following personal protective equipment (PPE) when caring for a patient with suspected or confirmed mpox infection: gown, gloves, eye protection and N95 (or comparable) respirator.
Is post-exposure prophylaxis recommended for health care workers who have been exposed to mpox?
Health care workers who have unprotected, high risk contact with patients with mpox may be eligible for post-exposure prophylaxis in consultation with public health authorities. Post-exposure prophylaxis involves receipt of vaccine, optimally within 4 days of exposure. Transmission of mpox virus from patients to health care workers has not occurred to date in this outbreak, lending support to the recommendation for post-exposure prophylaxis as the primary means for protecting health care workers.
What is the guidance for newborns in hospitals who may have been exposed to mpox during and after delivery?
Infants born to someone with suspected or confirmed mpox should undergo early bathing and post-exposure prophylaxis. While the optimal strategy for post-exposure prophylaxis of newborns has not been defined, Vaccinia Immune Globulin should be considered. Infants should also stay in a separate room and not have direct contact with parent (s) or caregivers infected with mpox. Breastfeeding should be delayed during the isolation period, and breastmilk should be pumped and discarded.
Page Last Updated: 10/3/2024

